Requests for Proposals
Maine TREE is seeking proposals to complete a post-harvest 100% tree inventory at Holt Research Forest (HRF). This effort will contribute to a repository of long-term ecological monitoring on-site, serving both regional landowners and the scientific community. Since the establishment of HRF in 1983, five 100% tree inventories of the study area have been completed. The last 100% timber inventory was completed in 2020, before a timber harvest at Holt Research Forest that same year. This post-harvest inventory will contribute to analysis of harvest impact.
Proposals are due on May 29 to [email protected]. Please direct questions to the same. Find complete requirements to the right.
Study Area
Grid System
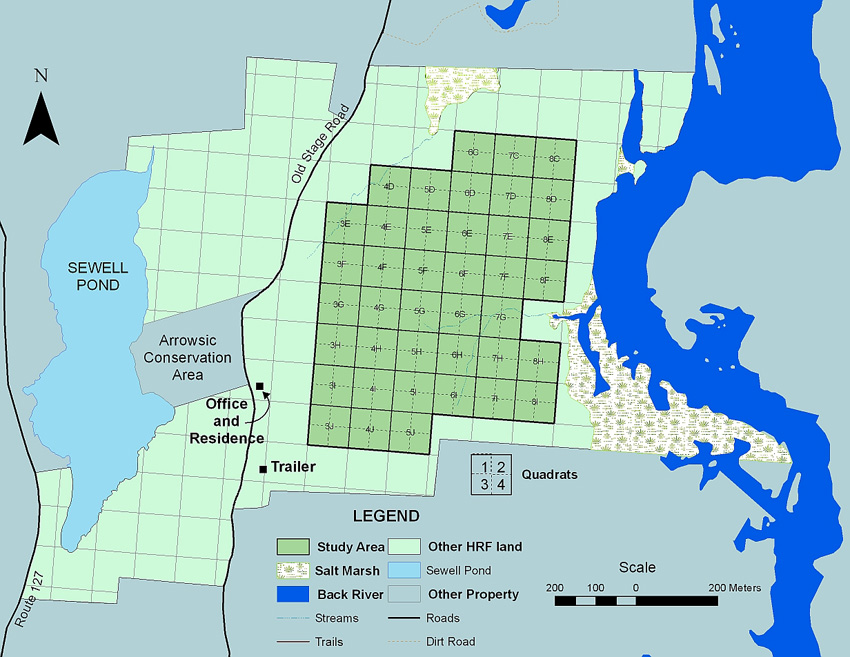
A grid system has been superimposed over the Holt Research Forest. The entire property is divided into 1-hectare “blocks.” The main study area consists of a central 40-ha (about 100 acres) area between Old Stage Road and the Back River to the east.
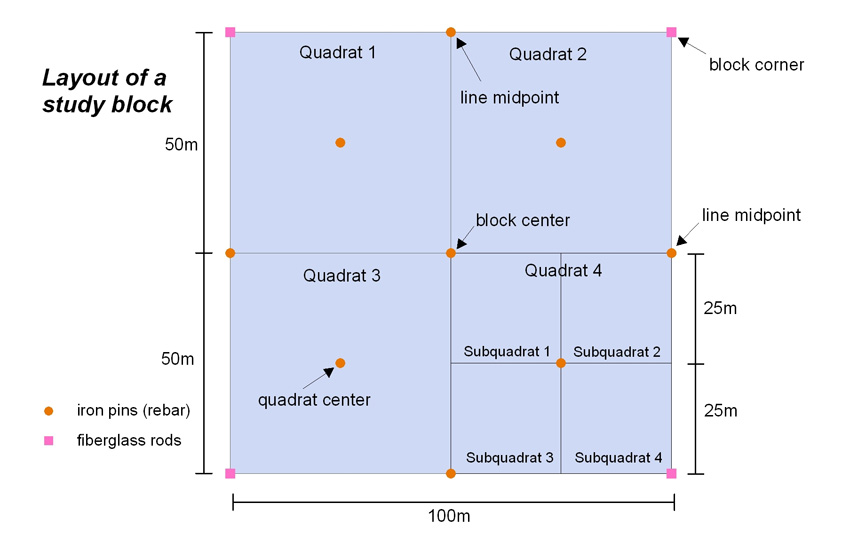
Within the study area, the blocks are further subdivided into 0.25 ha “quadrats” and 0.0625 ha “subquadrats.” The grid system provides excellent ground resolution as a “known” point at most 25 meters away.
Harvest Design
1987-88
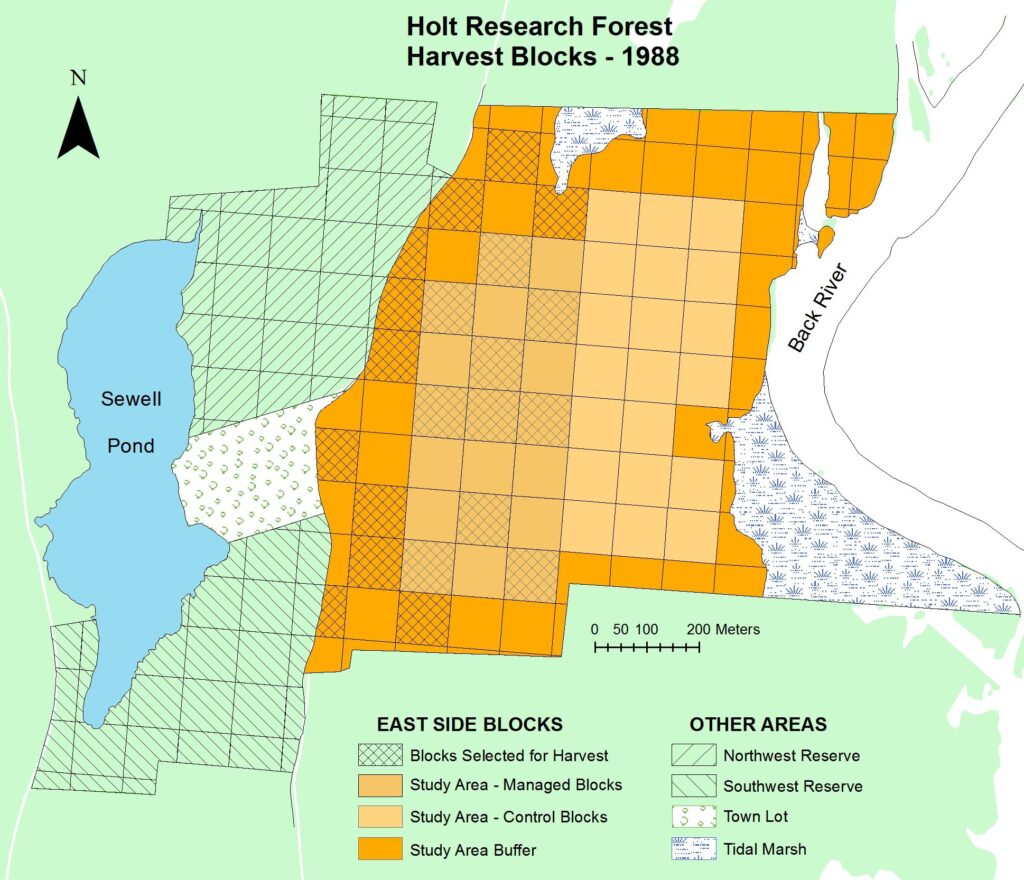
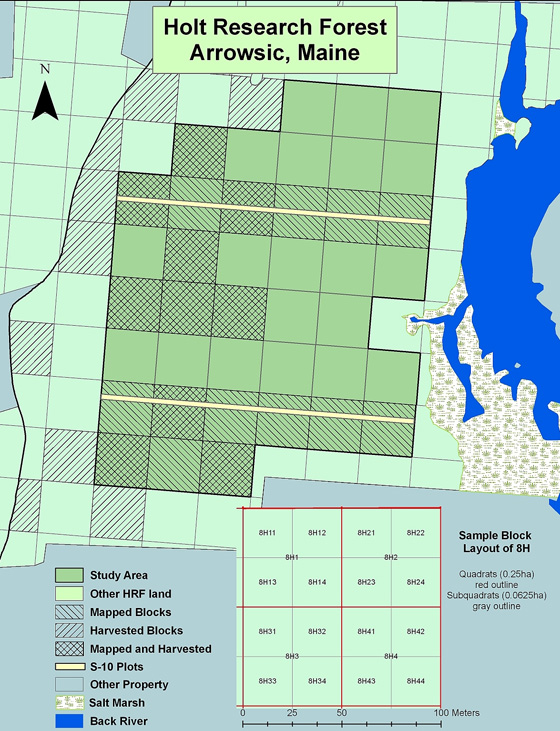
Within the 120 hectare (300 acre) Holt Research Forest, we selected a 40 hectare (100 acre) study area. The study area is located on the eastern half of the property, between Old Stage Road to the west and the Back River to the east. We divided the study area into 40 one-hectare (0.4 acre) blocks, then subdivided each block resulting in 160 50×50 meter quadrats, and 640 25×25 meter subquadrats. We bisected the study area along a north-south line into a “managed” half on the west and a “control” half on the east. We also established “buffer” blocks around the study area.
In the winter of 1987-88, we randomly selected and harvested 10 of the 1-ha blocks on the managed side. This harvest removed 44% of the basal area, including many large, poorly formed trees. It created openings in the canopy–harvest gaps–to release advanced regeneration and encourage new regeneration, thereby adding a new age class the forest. See Research – Forest Canopy Gaps for more details.
Thus we were on our way to meeting our forest management objectives to
- increase structural habitat diversity by adding a new vertical layer of vegetation, and
- arrive at a more balanced age-class distribution to generate an even flow of forest products over time.
We believed that the small gap sizes minimized the loss of near-term aesthetic appeal while enhancing the beauty of the forest over the long run. Subsequent monitoring and research on both the managed and control sides is giving us data to interpret the effects of forest management on various ecosystem components.
Methods & Findings
Land Use
Composition and structure of forest ecosystems are associated with physical factors (e.g., moisture, nutrients, and light) and biological factors (e.g., competition between plants and consumption by animals). Disturbances affect plants by altering physical and biological factors, but we often fail to appreciate how long these disturbances continue to influence the ecosystem. Sometimes our only clue to past disturbances may come from characteristics of the forest itself, long after visible signs of the disturbance have faded.
The land-use history of the Holt Research Forest is a case in point. It is bisected by a former property line that separated the “North Farm” from the “South Farm”. The farms differed in many respects, including the amount of land cleared for agriculture and cut for forest products, and the date of farm abandonment. This is reflected by composition and structure of the forest vegetation on either side of the line. For example, spinulose woodfern is far more abundant in the understory of the north, whereas trailing arbutus primarily occurs in the south. See Moore and Witham article published in Environmental History.

The historical development of two forest stands on either side of the line differed. In the northern stand, much of the white pine has big-limbed structure and wide early growth rings indicating that they were established under open conditions. They reached breast height (130 cm) from 1910 to 1930, while the red oak in this stand did not reach breast height until the 1940s. In contrast, the pines and oaks in the southern stand established more or less together between 1910 and 1930. In both stands, periods of light cutting are indicated by abrupt increases in radial growth. Historical census and tax data point to the North Farm being agriculturally (livestock and crops) active into the 1930s. This same data shows the South Farm with few animals and minimal agricultural production but owners with wood processing equipment such as portable shingle mills. This demonstrates that the majority of this property was historically a woodlot. What we observe today reflects these distinct land uses.
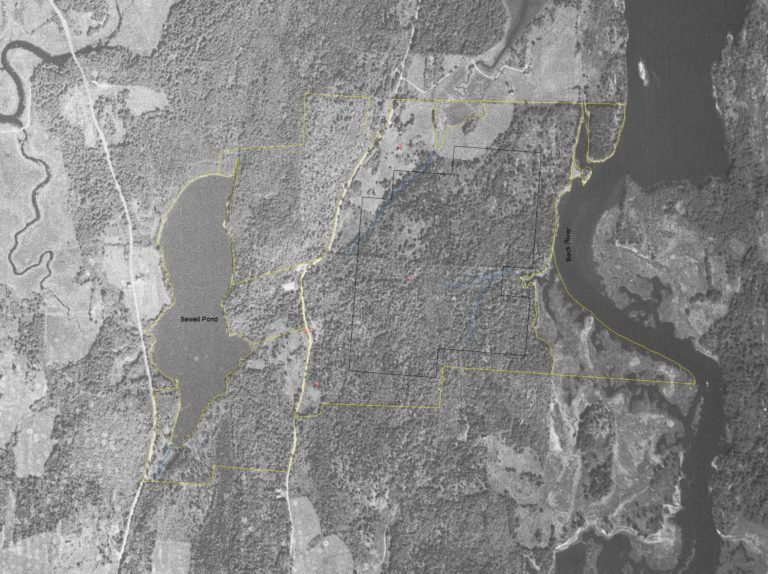
This air photo from 1940 illustrates the condition of the property with forest and agricultural lands visible. Yellow lines indicate the current property lines, black lines outline the study area, blue lines indicate ephemeral streams, and red dots are the location of old foundations. A distinct east/west property line is visible in the middle of the study area, this line still remains visible in photos today.
Forest Stand Structure and Composition

The Holt Research Forest study area comprises 38 stands 51% mixed stands growing on mesic soil, 10% mixed xeric, 8% deciduous mesic, 17% coniferous mesic, and 14% coniferous xeric. We have done three 100% inventories of the study area for all trees with dbh > 10cm (4in): pre-harvest (1984), immediate post-harvest (1988), and 8 years post-harvest (1996). Principal tree species, in order of both trees per hectare and total stand basal area, are eastern white pine, red maple, northern red oak, and red spruce. For a study area view of tree species abundance and basal area by hectare follow this link to a species distribution data map.
The winter 1987-88 harvest removed 44% of the basal area in the harvested 1-ha blocks. Volume and relative abundance of the large, rough white pines decreased from 44% to 38% of merchantable volume. Relative abundance red oak increased from 16% to 22% of cords/acre. The total volume removed was 210 cords of firewood, 304 cords of pulpwood, and 117 mbf of sawlogs. Between 1988 and 1996, all tree species gained in volume except the “other” softwood group in the control blocks. The latter suffered enough mortality over the eight years that it had a negative annual growth rate (-3% in cords/acre and -5% in board feet/acre). In contrast, red maple and red oak are grew at slightly over 6% annually in board feet /acre in the harvested blocks.
The sustainability of managed forests depends as much on the establishment and culture of seedlings and saplings as it does on the growth of the residual overstory trees. Since 1984 we have monitored the recruitment and development of new trees using several sampling designs, both in the forest as a whole, and in the canopy gaps. To date we have discovered that white pine, red maple, and red oak are all more abundant and taller in the canopy gaps than under the undisturbed canopy (control) adjacent to the gaps, considering both stump sprouts and seedlings.

Red maple and white pine regeneration are both more abundant and taller than red oak. This difference appears to be due to deer preferentially browsing the red oak seedlings and sprouts. 1993 was a major seed year for red oak. Clark (1996) followed the fate of oak seedlings that germinated following the 1993 mast year and found that while new seedlings were abundant under mature oaks and at the base of slopes, few new seedlings occurred in the canopy gaps. Because the only way for acorns to get into gap areas would be through animal (e.g., blue jays and chipmunks) dispersal, it is assumed that these animals chose not to cache acorns in the relatively open environment of the gaps.
In contrast, oak seedlings that were established prior to the harvests appear to grow well in gaps, but only if they are not browsed by deer. Thus, oak mast years probably are important in establishing oak seedlings under the forest canopy, but for those seedlings to make it into the canopy, they must be released from competition and protected from deer.
Forest Canopy Gaps

Much of our research at the Holt Research Forest focuses on vegetative and animal response to gaps–both natural and harvest–in the forest canopy. Response to gap size interests many natural resource professionals. Silviculture sometimes defines methods used to regenerate forests by the size of the gap a harvest produces. Foresters debate the size and arrangement of gaps needed to regenerate a given tree species or to achieve a desired age/size distribution. Wildlife biologists debate gap design and how to manage for a particular wildlife species or to maintain species richness.
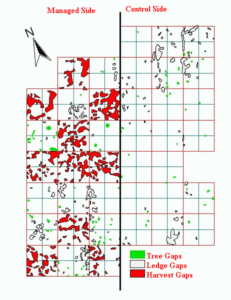
After the selective harvest in winter 1987-88, we inventoried and mapped all harvest gaps, plus naturally occurring ledge gaps (caused by the absence of canopy trees due to extremely shallow soils or exposed bedrock) and tree gaps (caused by the natural loss of trees in the canopy). Tree gaps covered only 0.7% and ledge gaps only 3.2% of the uncut forest whereas the harvest-created gaps covered 26.5% of the partially cut forest.
After four growing seasons, the forest floor vegetation of the three types of gaps differed significantly with junipers dominating ledge gaps, ferns and tree seedlings dominating tree gaps, and tree seedlings, forbs, and slash (downed woody material left from timber harvest) dominating harvest-created gaps. See Kimball et. al. for more details.
Tree Regeneration
Several methods are used to assess tree regeneration at HRF. We have sampled regeneration at several plot sizes with different strategies and size classes.
200 m² and 4 m² plots located on quadrat centers. In 200 m² plots all trees (saplings) 1.5-9.5 cm DBH are counted by species, condition and 1 cm size class. In 4 (located N, E, S, W) 4 m² plots all trees (seedlings and saplings) <1.5 cm DBH are counted by size class. Size classes are <0.1m tall; 0.1 – 0.49m tall; 0.5 – 2m tall; and >2m tall & <1.5cm DBH.
25 m² plots are located in 1997 canopy gaps and under canopy in similar soil types at the location of 1m² relevé plots (see Schumann, et al., 2003). All trees were counted by species and size class which included 0.5 – 2m tall; >2m tall & <1.5cm DBH; and one cm DBH classes from 1.5-9.5 cm. Additionally an assessment of damage is conducted, primarily focused on deer browsing but also insect damage such as white pine weevil.
S-10 plots are located in two west to east strips across the study area in the E and I blocks. Each strip is 10×600 m. Within this area all saplings (1.5-9.5 cm DBH) identified by species are measured and mapped to produce X,Y coordinates for each stem.
S-1 plots are the southernmost 1 m of the S-10 plots. The size classes measured are the same as 4 m² plots. Counts are completed each year in late July/early August at every 5th plot.

Birds
At the Holt Research Forest we censused birds year-round for many years and have been mapping bird territories during the breeding season since 1983. We have observed 124 species of birds, 35 of which breed here. In the forest ecosystem, birds are consumers of insects, distributors and predators of seeds, and sometimes are indicators of forest condition primarily reflecting the successional stage of the forest.

We are have studied the response of the bird community to the winter 1987-88 harvest, as well as long-term trends at the Holt Forest versus regional trends. Populations of some forest interior bird species (e.g., ovenbird, black-throated green warbler, eastern pewee) decreased in harvested areas but remained stable elsewhere. Early successional-stage species (e.g., common yellowthroat, white-throated sparrow) increased in harvested areas.
Five pairs of winter wrens, a species not previously observed at the Holt Forest, were a first-year post-harvest surprise. The wrens used the microhabitat created by slash and disappeared over several years as the slash decomposed.
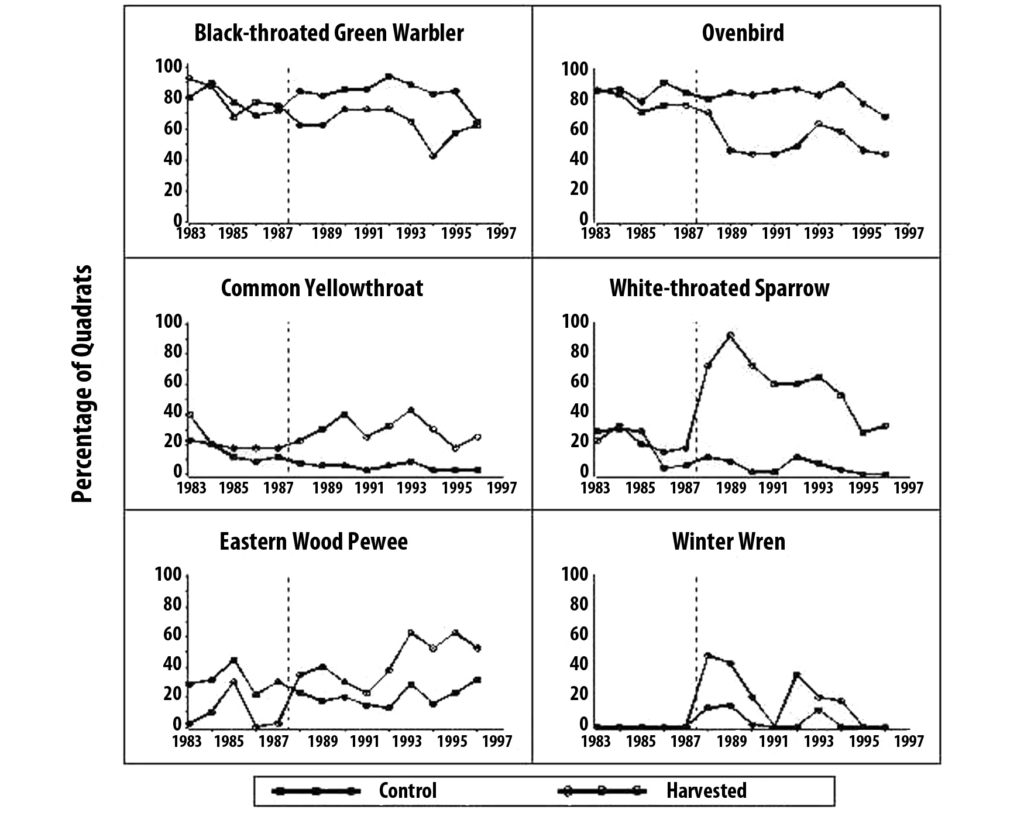
While the overall impact of timber harvesting on the bird community needs more in-depth analysis, our preliminary analyses indicate that the harvest has not dramatically altered the structure of the bird community within the 40-hectare study area. We may have minimized the impacts on birds by dispersing discontinuous small harvest gaps in only a portion of the forest.
Salamanders
At population densities of roughly 10,000 per hectare (more than 4,000 per acre) the red-backed salamander is by far the most abundant vertebrate on the Holt Research Forest. Given their abundance, habitat (forest floor litter), and diet (litter invertebrates), it is likely that they play a large role in forest ecology. For example, redbacks slow the rate of litter decomposition by preying on the invertebrates that shred leaf litter.
From 1983 to 1986 we conducted surveys designed to estimate population densities of redbacks. In 1987 we switched methodology to obtain a population index. Interpreting these data is complicated because the number of salamanders detected is very sensitive to rainfall patterns: more salamanders are near the surface during usually wet months such as May and during wet years. Nevertheless, redbacked salamander populations are probably reduced in the harvested portion of the forest compared to the control side. This topic will require further research because it is possible, albeit unlikely, that redback counts on the harvested area are not diminished; they may merely be living deeper in the soil and thus are harder to detect.
Small Mammals

Small mammals are among the most abundant vertebrate inhabitants of the Holt Research Forest and have wide-ranging effects on forest ecology. For example, several species are important predators of tree seeds and gypsy moths. Small mammal populations are monitored by live-trapping twice per year: in April to observe a low point in their annual fluxes, and August to observe a high point. Captures and recaptures of over 15,000 small mammals during 20 years have revealed some interesting patterns.
Cycles
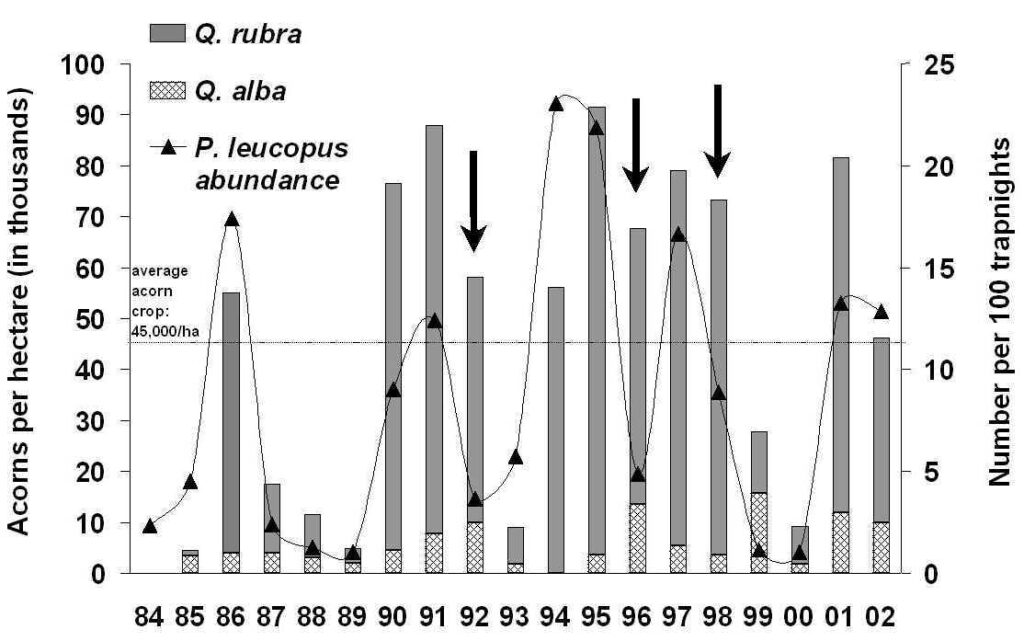
The connection between mice and mast is undisputed based on numerous studies. For example at the Holt Forest, based on 10 years of data, there were significant positive correlations between fall acorn crop production and the number and weights of white-footed mice (Peromyscus leucopus) the following spring. Their propensity for storing acorns during acorn-rich falls is probably responsible for this pattern.
With 20 years of data, it was found that summer populations of white-footed mice went through 4 abundance cycles with a cycle period of 4.0 years. Abundance always increased after large acorn crops and a low phase in the abundance cycle. However, in in 1992, 1996, and 1998 (abundance decreased following high abundance phases despite large acorn crops (see bold arrows in figure above). The index of fluctuation indicated a stronger fluctuation in Holt Forest P. leucopus than in populations in Indiana, Virginia, Ohio, and Pennsylvania. This periodicity may be mediated through as-yet unidentified factors such as predation.
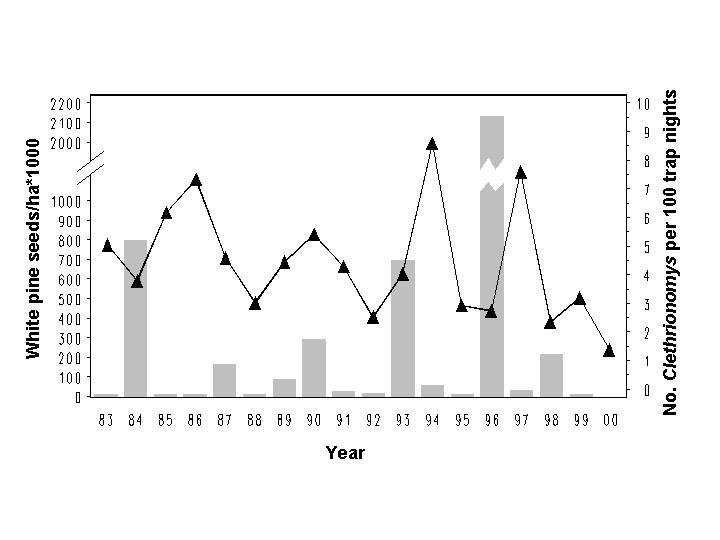
Red-backed voles (Clethrionomys gapperi) demonstrated a 3.6-year cycle. Abundance increased following three of four large white pine seed crops, leaving some doubt as to whether the white pine seed-vole connection was spurious or biologically meaningful. Regardless, cycling and density-dependence in Holt voles is consistent that found in vole populations in Scandinavia and elsewhere.
Habitat vs. Harvest
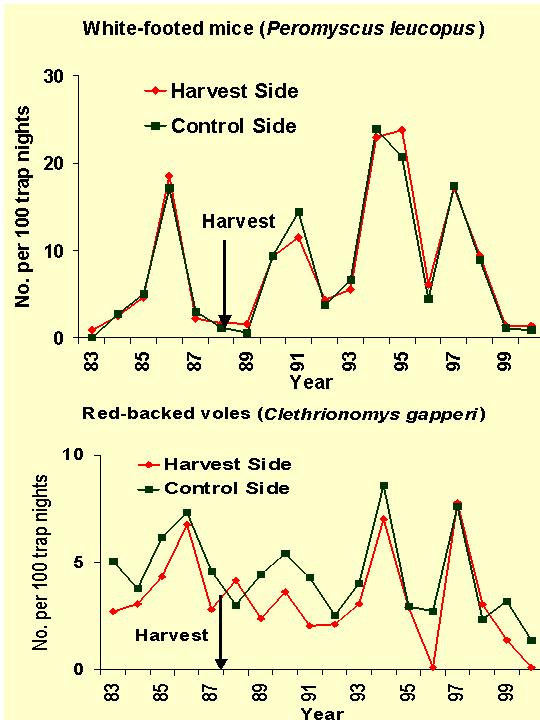
White-footed mouse cycles on the harvest and control sides of the forest corresponded closely across 18 years (1983-2000), but voles presented a different story. Harvest-side voles increased in the first year following harvest, then declined in the 2nd and 3rd years following harvest compared to control side voles. After five years, the harvest-side vole cycle again corresponded well with the control-side cycle. If cover is at issue, it is possible that voles found cover in first-year but not 2nd- and 3rd-year slash piles, then in subsequent years found cover in regeneration. We hope to test this hypothesis in the near future. The 1987-88 harvest coincided with a shift from predominantly northern flying squirrels on the study area to the southern flying squirrel. However, that this shift probably occurred because of regional population changes rather than changes to the forest structure.
Ancillary
Tree shelters for oak seedlings
White-tailed deer numbers appear to be on the rise on the Holt Research Forest. This could be due to a regional increase. Superimposed on this, we may have encouraged the entry of more deer into the Holt via the small harvest gaps created in 1987. Browsing is severe enough to suggest deer have had a negative impact on oak regeneration. In 2000, Al Kimball began comparing oak sapling growth between two treatments that might commonly be used by landowners to protect oak saplings from deer: (1) Bitrex (a bitter-tasting deer-repellent) and (2) tree shelters. Tree shelters provide physical protection from deer plus a micro-environment of warmth and carbon dioxide.
Research including investigators outside the faculty and students of the College of Forestry, Natural Sciences, and Agriculture at the University of Maine
- Since 1992 we have sent ticks collected from Holt Forest small mammals to Dr. Peter Rand and Dr. Robert Smith, Jr. of the Maine Medical Center Lyme Disease Research Lab. They are researching the the role of hosts, climate, and habitat factors affecting tick distribution in Maine.
- Dr. Joanne M. Sharpe began a long-term study of selected fern species on the Holt Research Forest in 1999. She is doing this in the context of a termperate/tropical comparison of long-term demographic and ecological trends in ferns. Her temperate sites are in Maine, and her tropical sites include the Luquillo Experimental Forest LTER and the Jobos Bay National Estuarine Research Reserve, both in Puerto Rico.
- In 1997 Genevieve Pullis and Kevin Boyle of the University of Maine Department of Resource Economics and Policy tested public perceptions of forest management activities at the Holt Research Forest. They found considerable differences among various segments of the public, but overall strong support for policies that balance timber harvesting and setting land aside from harvesting.
- In 1990 Bill Ostrofsky of the Maine Cooperative Forestry Research Unit and Al Kimball determined that trees released by the winter 1987-88 harvest did not show increased vigor.
- From 1993 through 1998, Nat Wheelwright of Bowdoin College assisted with a study of the reproductive effort of 15 species of herbs and shrubs.
Data
Data collected at the Holt Research Forest is publicly available via the Forest Ecosystem Monitoring Cooperative.
Explore data from the categories below and consider doing your own research!
Publications
Recent Publications
Campbell, S.P., J.W. Witham, and M.L. Hunter, Jr. 2012. Long-term changes in spatial distribution of birds responding to a group-selection timber harvest. Wildlife Society Bulletin 36 (2):313–327.
Ogawa, R., A. Mortelliti, J. W. Witham, and M. L. Hunter Jr. 2017. Demographic mechanisms linking tree seeds and rodent population fluctuations: insights from a 33-year study. Journal of Mammalogy.
Wang, G., N. T. Hobbs, S. H. Slade, J. F. Merritt, M. Hunter, N. A. Vessey, J. Witham, and A. Guillaumet. 2013. Comparative population dynamics of large and small mammals in the Northern Hemisphere: deterministic and stochastic forces. Ecography 36: 439–446.
Wood, C. M., J. W. Witham, and M. L. Hunter Jr. 2016. Climate-driven range shifts are stochastic processes at a local level: two flying squirrel species in Maine. Ecosphere 7(2):e01240. 10.1002/ecs2.1240
Earlier Publications
Campbell, S.P., J.W. Witham, and M.L. Hunter, Jr. 2007. A long-term study on the effects of a selection timber harvest on a forest bird community in Maine. Conservation Biology 21 (5): 1218–1229.
Campbell, S.P., J.W. Witham, and M.L. Hunter, Jr. 2010. Stochasticity as an alternative to deterministic explanations for patterns of habitat use in birds. Ecological Monographs 80(2):287-302.
Elias, S. P., J. W. Witham, and M. L. Hunter, Jr. 2006. A cyclic red-backed vole (Clethrionomys gapperi) and seedfall over 22 years in Maine. Journal of Mammalogy. 87: 440-445.
Elias, S. P., J. W. Witham, and M. L. Hunter. 2004. Peromyscus leucopus abundance and acorn mast: population fluctuations over 20 years. Journal of Mammalogy. 85:743-747.
Kimball, A.J., J.W. Witham, J.L. Rudnicky, A.S. White, M.L. Hunter, Jr. 1995. Harvest_created and natural canopy gaps in an oak_pine forest in Maine. Bull. Torrey Bot. Club 122: 115-123.
McKracken, K. E., J. W. Witham, and M. L. Hunter. 1999. Relationships between seed fall of 3 tree species and Peromyscus leucopus and Clethrionomys gapperi during 10 years in an oak-pine forest. Journal of Mammalogy 80:1288-1296.
Monti, L. M., M. L. Hunter, Jr. and J. W. Witham. 2000. An evaluation of the artificial cover object (ACO) method for monitoring populations of the Redback Salamander (Plethodon cinereus). Journal of Herpetology. 34(4):624-629.
Moore, E. H. and J. W. Witham. 1996. From forest to farm and back again: land use history as a dimension of ecological research in coastal Maine. Environmental History 1(3):50-69.
Meyer, S. R., R. G. Wagner, J. Witham, S. Norton, I. Fernandez, B. Wiersma, E. Small, J. Wilson, and A. Kimball. 2006. Long-term Forest Ecosystem Studies of the Cooperative Forestry Research Unit and the University of Maine. Pages 83-93 In L. C. Irland, A. E. Camp, J. C. Brissette, and Z. R. Donohew (eds). Long-term silvicultural & ecological studies: results for science and management. Yale University, School of Forestry & Environmental Studies, Global Institute of Sustainable Forestry Research Paper 005.
Plucinski, K. E. and M. L. Hunter, Jr. 2001. Spatial and temporal patterns of seed predation on three tree species in an oak-pine forest. Ecography 24: 309–317.
Schumann, M. E., A. S. White, and J. W. Witham. 2003. The effects of harvest-created gaps on plant species diversity, composition, and abundance in a Maine oak-pine forest. Forest Ecology and Management 176:543-561.
Small, M. F., and M. L. Hunter, Jr. 1988. Forest fragmentation and avian nest predation in forested landscapes. Oecologia 76 (1): 62-64.
Small, M. F., and M. L. Hunter, Jr. 1988. Response of passerines to abrupt forest-river and forest-powerline edges in Maine. Wilson Bulletin 101:77-83.
Wang, G., J. O. Wolff, S. H. Vessey, N. A. Slade, J. W. Witham, J. F. Merritt, M. L. Hunter, Jr. and S. P. Elias. 2009. Comparative population dynamics of Peromyscus leucopus in North America: influences of climate, food, and density dependence. Population Ecology 51:133–142.
White, A. S., J. W. Witham, M. L. Hunter, Jr., and A. J. Kimball. 1999. Relationship between plant species richness and biomass in a coastal Maine Quercus-Pinus forest. Journal of Vegetation Science 10:755-762.
Whitman, A., M. L. Hunter, Jr., and J. W. Witham. 1998. Age distribution of ramets of a forest herb, Wild Sarsaparilla, Aralia nudicaulis (Araliaceae). Canadian Field-Naturalist 112: 37- 44.
Witham, Jack with Nancy Coverstone and Renae Moran. 2004. Wild Apple Trees for Wildlife. Bulletin #7126. Habitats A Fact Sheet Series on Managing Lands for Wildlife. University of Maine Cooperative Extension. 8 pp. See UMCE website.
Witham, Jack with Nancy Coverstone and Lois Berg Stack. 2002. Understanding Ruby-throated Hummingbirds and Enhancing Their Habitat in Maine. Bulletin #7152. Habitats A Fact Sheet Series on Managing Lands for Wildlife. University of Maine Cooperative Extension. 12 pp. See UMCE website.
Witham, J. W. 1999. Northern Redback salamander. Pp. 66-70 in M. L. Hunter, Jr., A. J.K. Calhoun, M. McCollough (eds.) Maine Amphibians and Reptiles. University of Maine Press. Orono. 254 pp.
Witham, J. W. , M. L. Hunter, Jr., H. C. Tedford III, A. J. Kimball, A. S. White, and S. P. Elias. 1999. A Long term Study of an Oak-Pine Forest Ecosystem: a brief overview of the Holt Research Forest. Maine Agricultural and Forest Experiment Station Miscellaneous Publication 745. (Available as pdf)
Witham, J. W. and A. J. Kimball. 1996. Use of a geographic information system to facilitate analysis of spot mapping data. Journal of Field Ornithology 67(3):367-375.
Witham, J.W., E.H. Moore, M.L. Hunter, Jr., A.J. Kimball, and A.S. White. 1993. A Techniques Manual for the Holt Research Forest: A Long Term Study of an Oak_Pine Forest Ecosystem. Maine Agricultural Experiment Station Technical Bulletin 153. (Available as a pdf)
Witham, J. W. 1992. Redback salamander. Pp. 46_50 in M. L. Hunter, Jr., J. Albright and J. Arbuckle (eds.) The Amphibians and Reptiles of Maine. Maine Agric. Exp. Sta. Bull. 838. 174 pp.
Witham, J.W. and M.L. Hunter, Jr. 1992. Population trends of neotropical landbird migrants in northern coastal New England. Pp. 85_95. J. Hagan and D. Johnston, editors. Ecology and Conservation of Neotropical Migrant Landbirds. Smithsonian Institution Press, Washington, D.C., 605 pp.
Dissertations
- Campbell, S. 2007. The Long-term Effects of a Group-selection Timber Harvest on the Bird Community of an Oak-Pine Forest in Maine. Ph.D. dissertation, University of Maine, Orono.
- McCracken, K. E. 1996. Seed predation by small mammals on three species of trees in an oak-pine forest ecosystem. Ph.D. dissertation, University of Maine, Orono. 107 pp.
Master & Honor Theses
Schumann, M. E. 1999. The effects of harvest-created gaps on plant species diversity, composition, abundance, and growth in a Maine oak-pine forest. M.S. thesis, University of Maine, Orono. 179 pp.
Monti, L. M. 1997. Redback salamander habitat preferences in a Maine oak-pine forest. M. S. Thesis, University of Maine, Orono. 53 pp.
Clark, T. L. 1996. Stand development, growth patterns, and regeneration success of red oak in an oak-pine forest in southern Maine. M.S. thesis, University of Maine, Orono. 154 pp.
Gardiner. S. 1992. Effects of two soil types on ectomycorrhizal infection of white pine (Pinus strobus L.) seedlings. Honors Thesis, University of Maine, 48 p.
Whitman, A. A. 1992. Frugivory and seed dispersal of fleshy-fruiting plants in a northern temperate forest. M. S. Thesis, University of Maine, Orono. 214 pp.
Crowley, S. 1990. Habitat scaling and niche displacement of juvenile by adult red_backed voles (Clethrionomys gapperi). Honors Thesis, University of Maine.
Wood, M. L. 1987. The distribution of the salamander Plethodon cinereus relative to environmental characteristics in an oak_pine forest. Honors thesis, University of Maine.
Small, M. F. 1986. Response of songbirds and small mammals to powerline and river edges of Maine oak-pine forests. M. S. Thesis, University of Maine, Orono. 58 pp.
Use of Holt Research Forest Data
Boonstra, R. and C. J. Krebs. 2012. Population dynamics of red-backed voles (Myodes) in North America. Oecologia 168 (3): 601-620.
Haynes, K. J., A. M. Liebhold, T. M. Fearer, G. Wang, G. W. Norman, and D. M. Johnson. 2009. Spatial synchrony propagates through a forest food web via consumer–resource interactions. Ecology 90(11): 2974–2983.
Moore, J. D., & M. Ouellet. (2015). Questioning the use of an amphibian colour morph as an indicator of climate change. Global Change Biology 21(2): 566-571.
Use of Holt Research Forest Study Area
Lambert, A. M. and R. A.Casagrande. 2006. No Evidence of Fungal Endophytes in Native and Exotic Phragmites australis. Northeastern Naturalist 13(4):561–568.
Lambert, A. M. and R. A.Casagrande. 2007. Susceptibility of Native and Non-Native Common Reed to the Non-Native Mealy Plum Aphid (Homoptera: Aphididae) in North America. Environmental Entomology 36(2):451-457.
Li, X., Boyle, K. J., Holmes, T. P., & LaRouche, G. P. (2014). The effect of on-site forest experience on stated preferences for low-impact timber harvesting programs. Journal of Forest Economics 20(4): 348-362.
Lucas, R. W. 2007. Peeking into the black box: The structure and function of soil microbial communities in response to increasing nitrogen availability. PhD Dissertation, University of Pennsylvania, Philadelphia. 147pp. Dissertations available from ProQuest. Paper AAI3271782. http://repository.upenn.edu/dissertations/AAI3271782
Lucas, R.W. and B.B. Casper. 2008. Ectomycorrhizal Community and Ecosystem Functioning Following Simulated Atmospheric N Deposition. Soil Biology and Biochemistry 40:1662-1669.
Pullis, G. 1998. Public perceptions of forest ecosystem attributes and economic values for small, private woodlots with and without alternative timber harvesting. M. S. Thesis, University of Maine, Orono. 138 pp
Species Lists
Mammals
| Common Name | Scientific Name |
| Shrews and Moles: Insectivora | |
| Masked Shrew | Sorex cinereus |
| Short-tailed Shrew | Blarina brevicauda |
| Hairy-tailed Mole | Parascalops breweri |
| Star-nosed Mole | Condylura cristata |
| Rodents: Rodentia | |
| Eastern Chipmunk | Tamias striatus |
| Woodchuck | Marmota monax |
| Gray Squirrel | Sciuris carolinensis |
| Red Squirrel | Tamiasciuris hudsonicus |
| Southern flying Squirrel | Glaucomys volans |
| Northern flying Squirrel | Glaucomys sabrinus |
| Beaver | Castor canadensis |
| White-footed Mouse | Peromyscus leucopus |
| Red-backed Vole | Clethrionomys gapperi |
| Meadow Vole | Microtus pennsylvanicus |
| Muskrat | Ondatra zibethicus |
| Woodland jumping mouse | Napaeozapus insignis |
| Porcupine | Erethizon dorsatum |
| Carnivores: Carnivora | |
| Coyote | Canis latrans |
| Red Fox | Vulpes vulpes |
| Racoon | Procyon lotor |
| Fisher | Martes pennanti |
| Ermine | Mustela erminea |
| Long-tailed Weasel | Mustela frenata |
| Mink | Mustela vison |
| Striped Skunk | Mephitis mephitis |
| River Otter | Lutra canadensis |
| Hoofed Mammals: Artiodactyla | |
| White-tailed Deer | Odocoileus virginianus |
| Moose | Alces alces |
Amphibians & Reptiles
| Common Name | Scientific Name |
| TOADS AND FROGS: ANURA | |
| American Toad | Anaxyrus americanus |
| Gray Treefrog | Hyla versicolor |
| American Bullfrog | Lithobates catesbeiana |
| Green Frog | Lithobates clamitans |
| Pickerel Frog | Lithobates palustris |
| Northern Leopard Frog | Lithobates pipiens |
| Wood Frog | Lithobates sylvatica |
| Spring Peeper | Pseudacris crucifer |
| SALAMANDERS: CAUDATA | |
| Spotted Salamander | Ambystoma maculatum |
| Northern Two-lined Salamander | Eurycea bislineata |
| Four-toed Salamander | Hemidactylium scutatum |
| Red-spotted Newt | Notophthalmus viridescens |
| Eastern Red-backed Salamander | Plethodon cinereus |
| SNAKES: SQUAMATA | |
| Ringneck Snake | Diadophis punctatus |
| Eastern Milk Snake | Lampropeltis triangulum |
| Smooth Greensnake | Opheodrys vernalis |
| Northern Brownsnake | Storeria dekayi |
| Redbellied Snake | Storeria occipitomaculata |
| Common Gartersnake | Thamnophis sirtalis |
| TURTLES: TESTUDINES | |
| Snapping Turtle | Chelydra serpentina |
Birds
| Common Name | Scientific Name |
| ANATIDAE – Swans, Geese, and Ducks | |
| Snow Goose | Chen caerulescens |
| Canada Goose | Branta canadensis |
| Wood Duck | Aix sponsa |
| American Black Duck* | Anas rubripes |
| Mallard* | Anas platyrhynchos |
| Canvasback | Aythya valisineria |
| Bufflehead | Bucephala albeola |
| Common Goldeneye | Bucephala clangula |
| Hooded Merganser | Lophodytes cucullatus |
| Common Merganser | Mergus merganser |
| PHASIANIDAE – Pheasants, Grouse, & Turkey | |
| Ruffed Grouse* | Bonasa umbellus |
| Wild Turkey* | Meleagris gallopavo |
| COLUMBIDAE – Pigeons and Doves | |
| Mourning Dove* | Zenaida macroura |
| CUCULIDAE – Cuckoos | |
| Black-billed Cuckoo | Coccyzus erthropthalmus |
| Yellow-billed Cuckoo | Coccyzus americanus |
| CAPRIMULGIDAE – Nightjars | |
| Common Nighthawk | Chordeiles minor |
| Eastern Whip-poor-will | Antrostomus vociferus |
| APODIDAE – Swifts | |
| Chimney Swift | Chaetura pelagica |
| TROCHILIDAE – Hummingbirds | |
| Ruby-throated Hummingbird* | Archilochus colubris |
| SCOLOPACIDAE – Sandpipers | |
| Least Sandpiper | Calidrus minutilla |
| Semipalmated Sandpiper | Calidris pusilla |
| Wilson’s Snipe | Gallinago delicata |
| American Woodcock* | Scolopax minor |
| Greater Yellowlegs | Tringa melanoleuca |
| Lesser Yellowlegs | Tringa flavipes |
| LARIDAE – Gulls, Terns, and Skimmers | |
| Herring Gull | Larus argentatus |
| Great Black-backed Gull | Larus marinus |
| GAVIIDAE – Loons | |
| Common Loon | Gavia immer |
| PHALACROCORACIDAE – Cormorants | |
| Double-crested Cormorant | Phalacrocorax auritus |
| ARDEIDAE – Bitterns and Herons | |
| Great Blue Heron | Aredea herodias |
| Snowy Egret | Egretta thula |
| Green Heron | Butorides virescens |
| Black-crowned Night-heron | Nycticorax nycticorax |
| CATHARTIDAE – New World Vultures | |
| Turkey Vulture | Cathartes aura |
| PANDIONIDAE – Ospreys | |
| Osprey | Pandion haliaetus |
| ACCIPITRIDAE – Kites, Eagles, and Hawks | |
| Bald Eagle | Haliaeetus leucocephalus |
| Northern Harrier | Circus cyaneus |
| Sharp-shinned Hawk | Accipiter striatus |
| Cooper’s Hawk | Accipiter cooperii |
| Northern Goshawk* | Accipiter gentilis |
| Red-shouldered Hawk | Buteo lineatus |
| Broad-winged Hawk* | Buteo platypterus |
| Red-tailed Hawk | Buteo jamaicensis |
| STRIGIDAE – Typical Owls | |
| Great-horned Owl* | Bubo virginianus |
| Barred Owl* | Strix varia |
| Northern Saw-whet Owl | Aegolius acadicus |
| ALCEDINIDAE – Kingfishers | |
| Belted Kingfisher | Megaceryle alcyon |
| PICIDAE – Woodpeckers | |
| Red-bellied Woodpecker | Melanerpes carolinus |
| Yellow-bellied Sapsucker | Sphyrapicus varius |
| Downy Woodpecker* | Picoides pubescens |
| Hairy Woodpecker* | Picoides villosus |
| Northern Flicker | Colaptes auratus |
| Pileated Woodpecker* | Dryocopus pileatus |
| TYRANNIDAE – Tyrant Flycatchers | |
| Olive-sided Flycatcher | Contopus cooperi |
| Eastern Wood-Pewee* | Contopus virens |
| Least Flycatcher | Empidonax minimus |
| Eastern Phoebe* | Sayornis phoebe |
| Great Crested Flycatcher* | Myiarchus crinitus |
| Eastern Kingbird | Tyrannus tyrannus |
| LANIIDAE – Shrikes | |
| Northern Shrike | Lanius excubitor |
| VIREONIDAE – Vireos | |
| Blue-headed Vireo* | Vireo solitarius |
| Red-eyed Vireo* | Vireo olivaceus |
| CORVIDAE – Jays and Crows | |
| Blue Jay* | Cyanocitta cristata |
| American Crow | Corvus brachyrhynchos |
| Common Raven | Corvus corax |
| HIRUNDINIDAE – Swallows | |
| Tree Swallow | Tachycineta bicolor |
| Barn Swallow | Hirundo rustica |
| PARIDAE – Chickadees and Titmice | |
| Black-capped Chickadee* | Poecile atricapillus |
| Tufted Titmouse* | Baeolophus bicolor |
| SITTIDAE – Nuthatches | |
| Red-breasted Nuthatch* | Sitta canadensis |
| White-breasted Nuthatch* | Sitta carolinensis |
| CERTHIIDAE – Creepers | |
| Brown Creeper* | Certhia americana |
| TROGLODYTIDAE – Wrens | |
| House Wren | Troglodytes aedon |
| Winter Wren* | Troglodytes hiemalis |
| Marsh Wren | Cistothorus palustris |
| REGULIDAE – Kinglets | |
| Golden-crowned Kinglet* | Regulus satrapa |
| Ruby-crowned Kinglet | Regulus calendula |
| TURDIDAE – Thrushes | |
| Veery* | Catharus fuscescens |
| Swainson’s Thrush | Catharus ustulatus |
| Hermit Thrush* | Catharus guttatus |
| American Robin* | Turdus migratorius |
| MIMIDAE – Mockingbirds and Thrashers | |
| Gray Catbird | Dumetella carolinensis |
| STURNIDAE | |
| European Starling | Sturnus vulgaris |
| BOMBYCILLIDAE – Waxwings | |
| Cedar Waxwing* | Bombycilla cedrorum |
| FRINGILLIDAE – FRINGILLINE and CARDUELINE FINCHES | |
| Purple Finch* | Haemorhous purpureus |
| White-winged Crossbill | Loxia leucoptera |
| Pine Siskin | Spinus pinus |
| American Goldfinch* | Carduelis tristis |
| Evening Grosbeak* | Coccothraustes vespertinus |
| PARULIDAE – Wood Warblers | |
| Ovenbird* | Seiurus aurocapillus |
| Black-and-white Warbler* | Mniotilta varia |
| Tennessee Warbler | Oreothlypis peregrina |
| Nashville Warbler* | Oreothlypis ruficapilla |
| Mourning Warbler | Geothlypis philadelphia |
| Common Yellowthroat* | Geothlypis trichas |
| American Redstart | Setophaga ruticilla |
| Cape May Warbler | Setophaga tigrina |
| Northern Parula* | Setophaga americana |
| Magnolia Warbler | Setophaga magnolia |
| Bay-breasted Warbler | Setophaga castanea |
| Blackburnian Warbler* | Setophaga fusca |
| Chestnut-sided Warbler* | Setophaga pensylvanica |
| Blackpoll Warbler | Setophaga striata |
| Black-throated Blue Warbler* | Setophaga caerulescens |
| Palm Warbler | Setophaga palmarum |
| Pine Warbler* | Setophaga pinus |
| Yellow-rumped Warbler* | Setophaga coronata |
| Prairie Warbler | Setophaga discolor |
| Black-throated Green Warbler* | Setophaga virens |
| Canada Warbler* | Cardellina canadensis |
| Wilson’s Warbler | Cardellina pusilla |
| Yellow-breasted Chat | Icteria virens |
| EMBERIZIDAE – Towhees and Sparrows | |
| Eastern Towhee | Pipilo erythrophthalmus |
| American Tree Sparrow | Spizelloides arborea |
| Chipping Sparrow* | Spizella passerina |
| Field Sparrow | Spizella pusilla |
| Nelson’s Sparrow* | Ammodramus nelsoni |
| Fox Sparrow | Passerella iliaca |
| Song Sparrow* | Melospiza melodia |
| Lincoln’s Sparrow | Melospiza lincolnii |
| Swamp Sparrow | Melospiza georgiana |
| White-throated Sparrow* | Zonotrichia albicollis |
| White-crowned Sparrow | Zonotrichia leucophrys |
| Dark-eyed Junco* | Junco hyemalis |
| CARDINALIDAE – Tanagers, Cardinals, Grosbeaks, and Buntings | |
| Scarlet Tanager* | Piranga olivacea |
| Rose-breasted Grosbeak | Pheucticus ludovicianus |
| Indigo Bunting* | Passerina cyanea |
| ICTERIDAE – Blackbirds and Orioles | |
| Red-winged Blackbird* | Agelaius phoeniceus |
| Common Grackle | Quiscalus quiscula |
| Brown-headed Cowbird* | Molothrus ater |
| Baltimore Oriole* | Icterus galbula |
Vascular Plants
| Family | Species | * on study area |
| Lycopodiaceae | Diphasiastrum complanatum (L.) Holub | * |
| Lycopodiaceae | Huperzia lucidula (Michx.) Trevisan | * |
| Lycopodiaceae | Lycopodium annotinum L. | * |
| Lycopodiaceae | Lycopodium clavatum L. | * |
| Lycopodiaceae | Lycopodium obscurum L. | * |
| Equisetaceae | Equisetum pratense Ehrh. | * |
| Equisetaceae | Equisetum sylvaticum L. | * |
| Osmundaceae | Osmunda cinnamomea L. | * |
| Osmundaceae | Osmunda claytoniana L. | * |
| Osmundaceae | Osmunda regalis L. v. spectabilis (Willd.) Gray | * |
| Dennstaedtiaceae | Dennstaedtia punctilobula (Michx.) T. Moore | * |
| Dennstaedtiaceae | Pteridium aquilinum (L.) Kuhn v. latiusculum (Desv.) Heller | * |
| Thelypteridaceae | Phegopteris connectilis (Michx.) Watt | * |
| Thelypteridaceae | Thelypteris noveboracensis (L.) Nieuwl. | * |
| Thelypteridaceae | Thelypteris palustris Schott v. pubescens (Lawson) Fern. | * |
| Aspleniaceae | Asplenium trichomanes L. | * |
| Dryopteridaceae | Athyrium filix-femina (L.) Mertens v. angustum (Willd.) Lawson | * |
| Dryopteridaceae | Dryopteris carthusiana (Vill.) H.P. Fuchs | * |
| Dryopteridaceae | Dryopteris cristata (L.) Gray | * |
| Dryopteridaceae | Dryopteris marginalis (L.) Gray | * |
| Dryopteridaceae | Gymnocarpium dryopteris (L.) Newman | * |
| Dryopteridaceae | Onoclea sensibilis L. | * |
| Dryopteridaceae | Polystichum acrostichoides (Michx.) Schott | * |
| Pinaceae | Abies balsamea (L.) P. Mill. | * |
| Pinaceae | Picea rubens Sarg. | * |
| Pinaceae | Pinus resinosa Ait. | * |
| Pinaceae | Pinus rigida P. Mill. | * |
| Pinaceae | Pinus strobus L. | * |
| Pinaceae | Tsuga canadensis (L.) Carr. | * |
| Cupressaceae | Juniperus communis L. v. depressa Pursh | * |
| Ranunculaceae | Anemone quinquefolia L. | * |
| Ranunculaceae | Coptis trifolia (L.) Salisb. ssp. groenlandica (Oeder) Hultn | * |
| Ranunculaceae | Ranunculus acris L. | * |
| Ranunculaceae | Thalictrum pubescens Pursh | * |
| Berberidaceae | Berberis vulgaris L. | * |
| Papaveraceae | Corydalis sempervirens (L.) Pers. | * |
| Hamamelidaceae | Hamamelis virginiana L. | * |
| Myricaceae | Comptonia peregrina (L.) Coult. | * |
| Myricaceae | Myrica gale L. | |
| Myricaceae | Myrica pensylvanica Loisel. | * |
| Fagaceae | Fagus grandifolia Ehrh. | * |
| Fagaceae | Quercus alba L. | * |
| Fagaceae | Quercus rubra L. | * |
| Betulaceae | Alnus incana (L.) Moench ssp. rugosa (Du Roi) Clausen | * |
| Betulaceae | Alnus serrulata (Ait.) Willd. | |
| Betulaceae | Betula alleghaniensis Britt. | * |
| Betulaceae | Betula papyrifera Marsh. | * |
| Betulaceae | Betula populifolia Marsh. | * |
| Betulaceae | Corylus cornuta Marsh. | * |
| Betulaceae | Ostrya virginiana (P. Mill.) K. Koch | * |
| Caryophyllaceae | Cerastium fontanum Baumg. ssp. vulgare (Hartman) Greuter & Burdet | ? |
| Caryophyllaceae | Moehringia lateriflora (L.) Fenzl | |
| Polygonaceae | Persicaria hydropiper (L.) Opiz | |
| Polygonaceae | Persicaria sagittata (L.) H. Gross | * |
| Polygonaceae | Rumex acetosella L. | * |
| Clusiaceae | Hypericum canadense L. | |
| Clusiaceae | Hypericum perforatum L. | |
| Clusiaceae | Triadenum virginicum (L.) Raf. | * |
| Sarraceniaceae | Sarracenia purpurea L. | |
| Cistaceae | Lechea intermedia Britt. | |
| Violaceae | Viola blanda Willd. | |
| Violaceae | Viola blanda Willd. v. palustriformis Gray | |
| Violaceae | Viola cucullata Ait. | * |
| Violaceae | Viola lanceolata L. | * |
| Violaceae | Viola macloskeyi Lloyd ssp. pallens (DC.) M.S. Baker | * |
| Violaceae | Viola primulifolia L. | * |
| Violaceae | Viola sagittata Ait. v. ovata (Nutt.) T. & G. | |
| Violaceae | Viola sororia Willd. | |
| Violaceae | Viola X bissellii House (cucullata X sororia) | |
| Salicaceae | Populus grandidentata Michx. | * |
| Salicaceae | Populus tremuloides Michx. | * |
| Brassicaceae | Cardamine parviflora L. v. arenicola (Britt.) O.E. Schulz | * |
| Ericaceae | Chimaphila umbellata (L.) W. Bart. ssp. cisatlantica (Blake) Hultn | |
| Ericaceae | Corema conradii (Torr.) Loud. | |
| Ericaceae | Epigaea repens L. | * |
| Ericaceae | Gaultheria procumbens L. | * |
| Ericaceae | Gaylussacia baccata (Wangenh.) K. Koch | * |
| Ericaceae | Kalmia angustifolia L. | * |
| Ericaceae | Lyonia ligustrina (L.) DC. | * |
| Ericaceae | Moneses uniflora (L.) Gray | * |
| Ericaceae | Monotropa hypopithys L. | * |
| Ericaceae | Monotropa uniflora L. | * |
| Ericaceae | Pyrola americana Sweet | |
| Ericaceae | Pyrola elliptica Nutt. | * |
| Ericaceae | Rhododendron canadense (L.) Torr. | * |
| Ericaceae | Rhododendron groenlandicum (Oeder) Kron & Judd | * |
| Ericaceae | Vaccinium angustifolium Ait. | * |
| Ericaceae | Vaccinium corymbosum L. | * |
| Ericaceae | Vaccinium myrtilloides Michx. | |
| Primulaceae | Lysimachia quadrifolia L. | * |
| Primulaceae | Lysimachia terrestris (L.) B.S.P. | * |
| Primulaceae | Trientalis borealis Raf. | * |
| Grossulariaceae | Ribes glandulosum Grauer | * |
| Grossulariaceae | Ribes lacustre (Pers.) Poir. | * |
| Rosaceae | Amelanchier canadensis (L.) Medik. | * |
| Rosaceae | Amelanchier nantucketensis Bickn. | * |
| Rosaceae | Crataegus macrosperma Ashe | * |
| Rosaceae | Fragaria virginiana Duchesne | * |
| Rosaceae | Geum aleppicum Jacq. | |
| Rosaceae | Malus sylvestris P. Mill. | * |
| Rosaceae | Photinia melanocarpa (Michx.) Robertson & Phipps | * |
| Rosaceae | Photinia X floribunda (Lindl.) Robertson & Phipps | *? |
| Rosaceae | Potentilla canadensis L. | |
| Rosaceae | Potentilla simplex Michx. | * |
| Rosaceae | Prunus pensylvanica L. f. | |
| Rosaceae | Prunus serotina Ehrh. | * |
| Rosaceae | Prunus virginiana L. | * |
| Rosaceae | Rosa canina L. | |
| Rosaceae | Rosa nitida Willd. | |
| Rosaceae | Rosa virginiana P. Mill. | * |
| Rosaceae | Rubus allegheniensis Porter | * |
| Rosaceae | Rubus canadensis L. | *? |
| Rosaceae | Rubus flagellaris Willd. | * |
| Rosaceae | Rubus frondosus Bigelow | *? |
| Rosaceae | Rubus hispidus L. | * |
| Rosaceae | Rubus idaeus L. | * |
| Rosaceae | Rubus occidentalis L. | * |
| Rosaceae | Rubus pubescens Raf. | * |
| Rosaceae | Sorbus americana Marsh. | *? |
| Rosaceae | Spiraea alba Du Roi v. latifolia (Ait.) Dippel | * |
| Rosaceae | Spiraea tomentosa L. | |
| Fabaceae | Amphicarpaea bracteata (L.) Fern. | * |
| Fabaceae | Trifolium aureum Pollich | |
| Fabaceae | Trifolium pratense L. | |
| Fabaceae | Vicia tetrasperma (L.) Schreb. | * |
| Onagraceae | Circaea alpina L. | * |
| Onagraceae | Epilobium ciliatum Raf. | * |
| Onagraceae | Epilobium coloratum Biehler | |
| Onagraceae | Oenothera biennis L. | |
| Cornaceae | Cornus alternifolia L. f. | * |
| Cornaceae | Cornus canadensis L. | * |
| Santalaceae | Comandra umbellata (L.) Nutt. | * |
| Aquifoliaceae | Ilex verticillata (L.) Gray | * |
| Aquifoliaceae | Nemopanthus mucronatus (L.) Loes. | * |
| Polygalaceae | Polygala paucifolia Willd. | * |
| Sapindaceae | Acer pensylvanicum L. | * |
| Sapindaceae | Acer rubrum L. | * |
| Sapindaceae | Acer saccharum Marsh. | |
| Anacardiaceae | Toxicodendron rydbergii (Rydb.) Greene | * |
| Oxalidaceae | Oxalis stricta L. | * |
| Balsaminaceae | Impatiens capensis Meerb. | * |
| Apiaceae | Aralia hispida Vent. | |
| Apiaceae | Aralia nudicaulis L. | * |
| Apiaceae | Hydrocotyle americana L. | * |
| Apiaceae | Panax trifolius L. | * |
| Gentianaceae | Bartonia virginica (L.) B.S.P. | * |
| Apocynaceae | Apocynum androsaemifolium L. | * |
| Solanaceae | Solanum dulcamara L. | * |
| Convolvulaceae | Calystegia sepium (L.) R. Br. | ? |
| Lamiaceae | Galeopsis tetrahit L. | * |
| Lamiaceae | Leonurus cardiaca L. | * |
| Lamiaceae | Lycopus uniflorus Michx. | * |
| Lamiaceae | Prunella vulgaris L. | * |
| Lamiaceae | Scutellaria lateriflora L. | * |
| Callitrichaceae | Callitriche palustris L. | * |
| Oleaceae | Fraxinus americana L. | * |
| Scrophulariaceae | Chelone glabra L. | * |
| Scrophulariaceae | Melampyrum lineare Desr. | * |
| Scrophulariaceae | Nuttallanthus canadensis (L.) D.A. Sutton | |
| Scrophulariaceae | Verbascum thapsus L. | * |
| Scrophulariaceae | Veronica officinalis L. | * |
| Scrophulariaceae | Veronica serpyllifolia L. | * |
| Orobanchaceae | Orobanche uniflora L. | |
| Campanulaceae | Lobelia inflata L. | * |
| Rubiaceae | Galium labradoricum (Wieg.) Wieg. | |
| Rubiaceae | Galium trifidum L. | |
| Rubiaceae | Galium triflorum Michx. | |
| Rubiaceae | Houstonia caerulea L. | * |
| Rubiaceae | Mitchella repens L. | * |
| Caprifoliaceae | Diervilla lonicera P. Mill. | * |
| Caprifoliaceae | Linnaea borealis L. ssp. longiflora (Torr.) Hult_n | * |
| Caprifoliaceae | Lonicera canadensis Marsh. | * |
| Adoxaceae | Sambucus canadensis L. | * |
| Adoxaceae | Viburnum acerifolium L. | * |
| Adoxaceae | Viburnum dentatum L. v. lucidum Ait. | * |
| Adoxaceae | Viburnum nudum L. v. cassinoides (L.) Torr. & Gray | * |
| Asteraceae | Achillea millefolium L. | |
| Asteraceae | Arctium minus Bernh. | |
| Asteraceae | Aster acuminatus Michx. | * |
| Asteraceae | Aster lateriflorus (L.) Britt | * |
| Asteraceae | Aster macrophyllus L. | * |
| Asteraceae | Aster umbellatus P. Mill. | |
| Asteraceae | Erigeron annuus (L.) Pers. | |
| Asteraceae | Euthamia graminifolia (L.) Nutt. | * |
| Asteraceae | Hieracium aurantiacum L. | * |
| Asteraceae | Hieracium caespitosum Dumort. | * |
| Asteraceae | Hieracium canadense Michx. | |
| Asteraceae | Hieracium paniculatum L. | * |
| Asteraceae | Hieracium pilosella L. | |
| Asteraceae | Hieracium scabrum Michx. | |
| Asteraceae | Ionactis linariifolius (L.) Greene | |
| Asteraceae | Lactuca biennis (Moench) Fern. | |
| Asteraceae | Lactuca canadensis L. | |
| Asteraceae | Leontodon autumnalis L. | |
| Asteraceae | Leucanthemum vulgare Lam. | |
| Asteraceae | Prenanthes altissima L. | * |
| Asteraceae | Prenanthes nana (Bigelow) Torr. | * |
| Asteraceae | Solidago bicolor L. | |
| Asteraceae | Solidago canadensis L. | * |
| Asteraceae | Solidago juncea Ait. | |
| Asteraceae | Solidago nemoralis Ait. | |
| Asteraceae | Solidago puberula Nutt. | * |
| Asteraceae | Solidago rugosa P. Mill. | * |
| Araceae | Arisaema triphyllum (L.) Schott | * |
| Araceae | Calla palustris L. | |
| Juncaceae | Juncus brevicaudatus (Engelm.) Fern. | * |
| Juncaceae | Juncus effusus L. | * |
| Juncaceae | Juncus tenuis Willd. | * |
| Juncaceae | Luzula acuminata Raf. | * |
| Juncaceae | Luzula multiflora (Ehrh.) Lej. | * |
| Cyperaceae | Carex albicans Spreng. | * |
| Cyperaceae | Carex albicans Spreng v. emmonsii (Torr.) Rettig | |
| Cyperaceae | Carex bromoides Willd. | * |
| Cyperaceae | Carex brunnescens (Pers.) Poir. ssp. sphaerostachya (Tuckerman) Kalela | * |
| Cyperaceae | Carex crinita Lam. | * |
| Cyperaceae | Carex disperma Dewey | |
| Cyperaceae | Carex echinata Murr. | * |
| Cyperaceae | Carex echinata Murr. | * |
| Cyperaceae | Carex folliculata L. | * |
| Cyperaceae | Carex gracillima Schwein. | * |
| Cyperaceae | Carex intumescens Rudge | * |
| Cyperaceae | Carex laxiculmis Schwein. | * |
| Cyperaceae | Carex laxiflora Lam. | * |
| Cyperaceae | Carex leptalea Wahlenb. | * |
| Cyperaceae | Carex lurida Wahlenb. | * |
| Cyperaceae | Carex pallescens L. | * |
| Cyperaceae | Carex projecta Mackenzie | * |
| Cyperaceae | Carex stipata Willd. | * |
| Cyperaceae | Carex trisperma Dewey | * |
| Cyperaceae | Carex umbellata Willd. | |
| Cyperaceae | Carex vulpinoidea Michx. | * |
| Cyperaceae | Eleocharis acicularis (L.) Roemer & J.A. Schultes | * |
| Cyperaceae | Eleocharis tenuis (Willd.) J.A. Schultes | |
| Cyperaceae | Scirpus cyperinus (L.) Kunth | |
| Cyperaceae | Scirpus microcarpus J. & K. Presl | |
| Poaceae | Anthoxanthum odoratum L. | * |
| Poaceae | Brachyelytrum septentrionale (Babel) G. Tucker | * |
| Poaceae | Dactylis glomerata L. | * |
| Poaceae | Danthonia spicata (L.) Roemer & J.A. Schultes | * |
| Poaceae | Deschampsia flexuosa (L.) Trin. | * |
| Poaceae | Glyceria striata (Lam.) A.S. Hitchc. | * |
| Poaceae | Oryzopsis asperifolia Michx. | * |
| Poaceae | Oryzopsis pungens (Spreng.) A.S. Hitchc. | * |
| Poaceae | Phleum pratense L. | * |
| Sparganiaceae | Sparganium eurycarpum Gray | * |
| Liliaceae | Clintonia borealis (Ait.) Raf. | * |
| Liliaceae | Lilium philadelphicum L. | * |
| Liliaceae | Maianthemum canadense Desf. | * |
| Liliaceae | Maianthemum racemosum (L.) Link | * |
| Liliaceae | Maianthemum trifolium (L.) Sloboda | |
| Liliaceae | Medeola virginiana L. | * |
| Liliaceae | Polygonatum pubescens (Willd.) Pursh | |
| Liliaceae | Uvularia sessilifolia L. | * |
| Iridaceae | Iris versicolor L. | * |
| Iridaceae | Sisyrinchium montanum Greene v. crebrum Fern. | |
| Smilacaceae | Smilax herbacea L. | * |
| Orchidaceae | Corallorrhiza maculata (Raf.) Raf. | * |
| Orchidaceae | Cypripedium acaule Ait. | * |
| Orchidaceae | Goodyera tesselata Lodd. | * |
| Orchidaceae | Platanthera clavellata (Michx.) Luer | * |
| Orchidaceae | Platanthera grandiflora (Bigelow) Lindl. | * |
Only scientific species names are provided. Plant list based on 1983 Revised Checklist of the Vascular Plants of Maine. Bulletin of the Josselyn Botanical Society Number 11.
